The matter around us is present in the form of elements, compounds and mixtures and the elements contain atoms of only one type.
At present, 118 elements are known to us. All these have different properties. Out of these 118, only 94 are naturally occurring.
There are different elements were being discovered; scientists gathered more and more information about the properties of these elements. They found it difficult to organize all that was known about the elements.
We have learnt across instances of organisation based on some properties. For example, in a shop, soaps are kept together at one place while biscuits are kept together elsewhere. Even among soaps, bathing soaps are stacked separately from washing soaps. Similarly, scientists made several attempts to classify elements according to their properties and obtain an orderly arrangement out of chaos.
The classification of the elements resulted in grouping the then known elements as metals and non-metals.
After the rejection of Newlands’ Law of Octaves, many scientists continued to search for a pattern that correlated the properties of elements with their atomic masses.
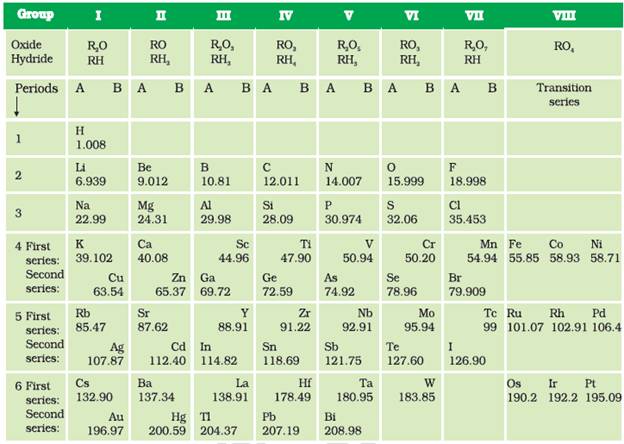
The main credit for classifying elements goes to Dmitri Ivanovich Mendeléev, a Russian chemist. He was the most important contributor to the early development of a Periodic Table of elements wherein the elements were arranged on the basis of their fundamental property, the atomic mass, and also on the similarity of chemical properties.
Mendeléev's Periodic Table contains vertical columns called ‘groups’ and horizontal rows called ‘periods’.
In 1913, Henry Moseley showed that the atomic number (symbolised as Z) of an element is a more fundamental property than its atomic mass. Accordingly, Mendeléev's Periodic Law was modified and atomic number was adopted as the basis of Modern Periodic Table and the Modern Periodic Law can be stated as follows:
‘Properties of elements are a periodic function of their atomic number.’
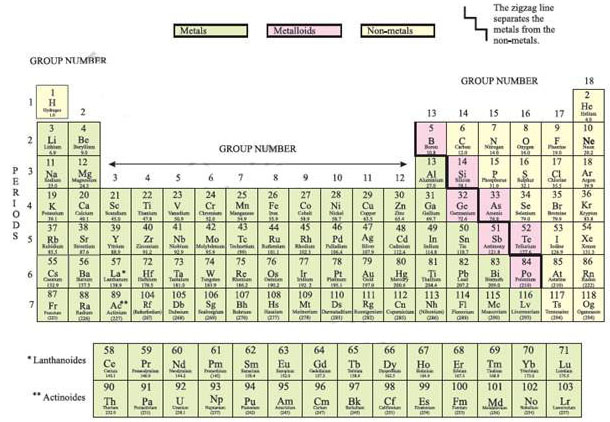
The Modern Periodic Table takes care of three limitations of Mendléev's Periodic Table.
The Modern Periodic Table has 18 vertical columns known as ‘groups’ and 7 horizontal rows known as ‘periods’. In the Periodic Table signify an identical outershell electronic configuration. On the other hand, the number of shells increases as we go down the group.
There is an anomaly when it comes to the position of hydrogen because it can be placed either in group 1 or group 17 in the first period.
Atoms of different elements with the same number of occupied shells are placed in the same period. Na, Mg, Al, Si, P, S, Cl and Ar belong to the third period of the Modern Periodic Table, since the electrons in the atoms of these elements are filled in K, L and M shells.
Each period marks a new electronic shell getting filled.
For example,
K Shell – 2 x (1)2 = 2, hence the first period has 2 elements.
L Shell – 2 x (2)2= 8, hence the second period has 8 elements.
The third, fourth, fifth, sixth and seventh periods have 8, 18, 18, 32 and 32 elements respectively.
The position of an element in the Periodic Table tells us about its chemical reactivity.
Trends in the modern periodic table
Valency : As you know, the valency of an element is determined by the number of valence electrons present in the outermost shell of its atom.
Atomic size: The term atomic size refers to the radius of an atom. The atomic size may be visualised as the distance between the centre of the nucleus and the outermost shell of an isolated atom. The atomic radius of hydrogen atom is 37 pm (picometre, 1 pm = 10-12m).
Metallic and Non-metallic Properties
The metals like Na and Mg are towards the left-hand side of the Periodic Table while the non-metals like sulphur and chlorine are found on the right-hand side.
In the middle silicon, which is classified as a semi-metal or metalloid because it exhibits some properties of both metals and non-metals.
In the Modern Periodic Table, a zig-zag line separates metals from non-metals. The borderline elements – boron, silicon, germanium, arsenic, antimony, tellurium and polonium – are intermediate in properties and are called metalloids or semi-metals.
Non-metals, on the other hand, are electronegative. They tend to form bonds by gaining electrons.
The electro-negativity show, non-metals are found on the right-hand side of the Periodic Table towards the top.
