The ionic compounds have high melting and boiling points and conduct electricity in solution or in the molten state. The atomic number of carbon is 6.
In the case of carbon, it has four electrons in its outermost shell and needs to gain or lose four electrons to attain noble gas configuration. If it were to gain or lose electrons −
It could gain four electrons forming C4− anion. But it would be difficult for the nucleus with six protons to hold on to ten electrons, that is, four extra electrons.
It could lose four electrons forming C4+ cation. But it would require a large amount of energy to remove four electrons leaving behind a carbon cation with six protons in its nucleus holding on to just two electrons.
Methane is widely used as a fuel and is a major component of bio-gas and Compressed Natural Gas (CNG). It is also one of the simplest compounds formed by carbon.
The numbers of carbon compounds whose formulae are known to chemists was recently estimated to be in millions! This outnumbers by a large margin the compounds formed by all the other elements put together. The nature of the covalent bond enables carbon to form a large number of compounds. Two factors noticed in the case of carbon are −
Carbon has the unique ability to form bonds with other atoms of carbon, giving rise to large molecules. This property is called catenation. These compounds may have long chains of carbon, branched chains of carbon or even carbon atoms arranged in rings.
Since carbon has a valency of four, it is capable of bonding with four other atoms of carbon or atoms of some other mono-valent element. Compounds of carbon are formed with oxygen, hydrogen, nitrogen, sulphur, chlorine and many other elements giving rise to compounds with specific properties which depend on the elements other than carbon present in the molecule.
Three valencies of each carbon atom remain unsatisfied, so each is bonded to three hydrogen atoms giving:
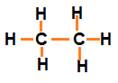
The valencies of all the atoms are satisfied by single bonds between them. Such carbon compounds are called saturated compounds. These compounds are normally not very reactive.
We mentioned the carbon compounds methane, ethane and propane, containing respectively 1, 2 and 3 carbon atoms. Such ‘chains’ of carbon atoms can contain many more carbon atoms.
All these carbon compounds which contain only carbon and hydrogen are called hydrocarbons. Among these, the saturated hydrocarbons are called alkanes. The unsaturated hydrocarbons which contain one or more double bonds are called alkenes. Those containing one or more triple bonds are called alkynes.
The heteroatoms and the group containing these confer specific properties to the compound, regardless of the length and nature of the carbon chain and hence are called functional groups.
The names of compounds in a homologous series are based on the name of the basic carbon chain modified by a “prefix” “phrase before” or “suffix” “phrase after” indicating the nature of the functional group Naming a carbon compound can be done by the following method −
Identify the number of carbon atoms in the compound. A compound having three carbon atoms would have the name propane.
In case a functional group is present, it is indicated in the name of the compound with either a prefix or a suffix
If the name of the functional group is to be given as a suffix, and the suffix of the functional group begins with a vowel a, e, i, o, u, then the name of the carbon chain is modified by deleting the final ‘e’ and adding the appropriate suffix.
If the carbon chain is unsaturated, then the final ‘ane’ in the name of the carbon chain is substituted by ‘ene’ or ‘yne’
Nomenclature of organic compounds
|
Class of compounds |
Prefix/Suffix |
Example |
|
1. Halo alkane |
Prefix-chloro, bromo, etc. |
Chloropropane 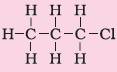
|
|
2. Alcohol |
Suffix – ol |
Propanol 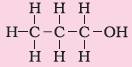
|
|
3. Aldehyde |
Suffix – al |
Propanal 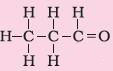
|
|
4. Ketone |
Suffix – one |
Propanone 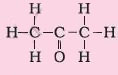
|
|
5. Carboxylic acid |
Suffix – oic acid |
Propanoic acid 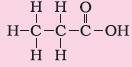
|
|
6. Alkenes |
Suffix – ene |
Propene 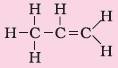
|
|
7. Alkynes |
Suffix – yne |
Propyne 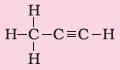
|
We Learnt about some of the chemical properties of carbon compounds. Since most of the fuels we use are either carbon or its compounds, we shall first study combustion.
The Carbon, in all its allotropic forms, burns in presence of oxygen to give carbon dioxide along with the release of heat and light. Most carbon compounds also release a large amount of heat and light on burning.
C + O2 → CO2 + heat and light
CH4 + O2 → CO2 + H2O + heat and light
Saturated and unsaturated carbon compounds
Saturated hydrocarbons will generally give a clean flame while, unsaturated carbon compounds will give a yellow flame with lots of black smoke.
Carbon compounds can be easily oxidised on combustion.
CH3 - CH2OH Alkaline KMnO4 + Heat/Or acidified K2Cr2O2> CH3COOH
These substances are known as oxidising agents.
Alkaline potassium permanganate or acidified potassium dichromate are oxidising alcohols to acids, that is, adding oxygen to the starting material. Hence they are known as oxidising agents.
Unsaturated hydrocarbons add hydrogen in the presence of catalysts such as palladium or nickel to give saturated hydrocarbons. Catalysts are substances that cause a reaction to occur or proceed at a different rate without the reaction itself being affected.
Saturated hydrocarbons are fairly unreactive and are inert in the presence of most reagents. However, in the presence of sunlight, chlorine is added to hydrocarbons in a very fast reaction. Chlorine can replace the hydrogen atoms one by one. It is called a substitution reaction because one type of atom or a group of atoms takes the place of another.
There are many carbon compounds are invaluable to us. Here we learn about two commercially important compounds – ethanol and ethanoic acid.
Ethanol is a liquid at room temperature. Ethanol is commonly called alcohol and is the active ingredient of all alcoholic drinks. In addition, because it is a good solvent, it is also used in medicines such as tincture iodine, cough syrups, and many tonics.
Ethanol is also soluble in water in all proportions. Consumption of small quantities of dilute ethanol causes drunkenness. Even though this practice is condemned, it is a socially widespread practice.
However, intake of even a small quantity of pure ethanol (called absolute alcohol).
Reactions of Ethanol
1) Reaction with sodium
2Na + 2CH3CH2OH → 2CH3CH2O – Na + H2
Alcohols react with sodium leading to the evolution of hydrogen. With ethanol, the other product is sodium ethoxide.
2) Reaction to give unsaturated hydrocarbon: Heating ethanol at 443 K with excess concentrated sulphuric acid results in the dehydration of ethanol to give ethane –
CH3 – CH2OH Hot Conc.→ H2SO4> CH2 = CH2 + H2oThe concentrated sulphuric acid can be regarded as a dehydrating agent which removes water from ethanol.
The ethanoic acid is commonly called acetic acid and belongs to a group of acids called carboxylic acids.
The melting point of pure ethanoic acid is 290 K and hence it often freezes during winter in cold climates.
The group of organic compounds called carboxylic acids are obviously characterized by their acidic nature. However, unlike mineral acids like HCl, which are completely ionised, carboxylic acids are weak acids.
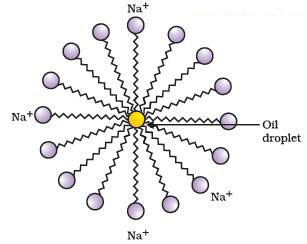
As we know that the soap used in cleaning. The molecules of soap are sodium or potassium salts of long-chain carboxylic acids. The ionic-end of soap interacts with water while the carbon chain interacts with oil. The soap molecules, thus form structures called micelles.
The soap micelle thus helps in pulling out the dirt in water and we can wash our clothes clean.
While bathing that foam is formed with difficulty and an insoluble substance (scum) remains after washing with water.
This problem is overcome by using another class of compounds called detergents as cleansing agents. Detergents are generally sodium salts of sulphonic acids or ammonium salts with chlorides or bromides ions, etc. Both have long hydrocarbon chain. The charged ends of these compounds do not form insoluble precipitates with the calcium and magnesium ions in hard water.
Detergents are usually used to make shampoos and products for cleaning clothes.
