An electron is represented as ‘e—’ and a proton as ‘p+’. The mass of a proton is taken as one unit and its charge as plus one. The mass of an electron is considered to be negligible and its charge is minus one.
An atom was composed of protons and electrons, mutually balancing their charges. It also appeared that the protons were in the interior of the atom, for whereas electrons could easily be removed off but not protons.
The atom was indivisible and indestructible by the Dalton's atomic theory. But the discovery of two fundamental particles (electrons and protons) inside the atom, led to the failure of this aspect of Dalton's atomic theory. It was then considered necessary to know how electrons and protons are arranged within an atom. For explaining this, many scientists proposed various atomic models.
J.J. Thomson was the first one to propose a model for the structure of an atom.
Thomson proposed the model of an atom to be similar to that of a Christmas pudding. The electrons, in a sphere of positive charge, were like currants (dry fruits) in a spherical Christmas pudding.
Thomson proposed that:
An atom consists of a positively charged sphere and the electrons are embedded in it.
The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral.
Ernest Rutherford was interested in knowing how the electrons are arranged within an atom.
On the basis of his experiment, Rutherford put forward the nuclear model of an atom, which had the following features:
There is a positively charged centre in an atom called the nucleus. Nearly all the mass of an atom resides in the nucleus.
The electrons revolve around the nucleus in circular paths.
The size of the nucleus is very small as compared to the size of the atom.
Drawbacks of Rutherford's model of the atom
The revolution of the electron in a circular orbit is not expected to be stable. Any particle in a circular orbit would undergo acceleration. During acceleration, charged particles would radiate energy. Thus, the revolving electron would lose energy and finally fall into the nucleus.
In order to overcome the objections raised against Rutherford's model of the atom, Neils Bohr put forward the following postulates about the model of an atom:
Only certain special orbits known as discrete orbits of electrons, are allowed inside the atom.
While revolving in discrete orbits the electrons do not radiate energy.
These orbits or shells are called energy levels. Energy levels in an atom are here.
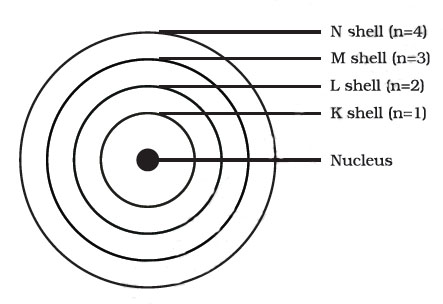
These orbits or shells are represented by the letters K,L,M,N,... or the numbers, n=1,2,3,4,....
In 1932, J. Chadwick discovered another subatomic particle which had no charge and a mass nearly equal to that of a proton. It was eventually named as neutron. Neutrons are present in the nucleus of all atoms, except hydrogen. In general, a neutron is represented as ‘n’. The mass of an atom is therefore given by the sum of the masses of protons and neutrons present in the nucleus.
The distribution of electrons into different orbits of an atom was suggested by Bohr and Bury.
The following rules are followed for writing the number of electrons in different energy levels or shells:
The maximum number of electrons present in a shell is given by the formula 2n2, where ‘n’ is the orbit number or energy level index, 1,2,3,....
The maximum number of electrons that can be accommodated in the outermost orbit is 8.
Electrons are not accommodated in a given shell, unless the inner shells are filled. That is, the shells are filled in a step-wise manner.
The electrons present in the outermost shell of an atom are known as the valence electrons.
From the Bohr-Bury scheme, we also know that the outermost shell of an atom can accommodate a maximum of 8 electrons. It was observed that the atoms of elements, completely filled with 8 electrons in the outermost shell show little chemical activity.
An outermost-shell, which had eight electrons was said to possess an octet. Atoms would thus react, so as to achieve an octet in the outermost shell. If the number of electrons in the outermost shell of an atom is close to its full capacity, then valency is determined in a different way.
Therefore, an atom of each element has a definite combining capacity, called its valency.
The atomic number is defined as the total number of protons present in the nucleus of an atom.
The number of protons of an atom, which determines its atomic number. It is denoted by ‘Z’. All atoms of an element have the same atomic number, Z. In fact, elements are defined by the number of protons they possess. For hydrogen, Z = 1, because in hydrogen atom, only one proton is present in the nucleus.
The mass number is defined as the sum of the total number of protons and neutrons present in the nucleus of an atom. It is denoted by ‘A’. In the notation for an atom, the atomic number, mass number and symbol of the element are to be written as:

For example, nitrogen is written as 14/7N.
In nature, a number of atoms of some elements have been identified, which have the same atomic number but different mass numbers.
Isotopes are defined as the atoms of the same element, having the same atomic number but different mass numbers. Therefore, we can say that there are three isotopes of hydrogen atom, namely protium, deuterium and tritium.
Many elements consist of a mixture of isotopes. Each isotope of an element is a pure substance. The chemical properties of isotopes are similar but their physical properties are different.
Applications
Since the chemical properties of all the isotopes of an element are the same, normally we are not concerned about taking a mixture. But some isotopes have special properties which find them useful in various fields. Some of them are :
An isotope of uranium is used as a fuel in nuclear reactors.
An isotope of cobalt is used in the treatment of cancer.
An isotope of iodine is used in the treatment of goitre.
The number of protons in these atoms is different, but the mass number of both these elements is 40. That is, the total number of nucleons is the same in the atoms of this pair of elements. Atoms of different elements with different atomic numbers, which have the same mass number, are known as isobars.
