There are two laws of chemical combination were established after much experimentations by Lavoisier and Joseph L. Proust.
Law of conservation of mass states that mass can neither be created nor destroyed in a chemical reaction.
The many compounds were composed of two or more elements and each such compound had the same elements in the same proportions, irrespective of where the compound came from or who prepared it.
The law of constant proportions which is also known as the law of definite proportions. This law was stated by Proust as “In a chemical substance the elements are always present in definite proportions by mass”.
According to Dalton's atomic theory, all matter, whether an element, a compound or a mixture is composed of small particles called atoms. The postulates of this theory may be stated as follows:
All matter is made of very tiny particles called atoms, which participate in chemical reactions.
Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction.
Atoms of a given element are identical in mass and chemical properties.
Atoms of different elements have different masses and chemical properties.
Atoms combine in the ratio of small whole numbers to form compounds.
The relative number and kinds of atoms are constant in a given compound.
The building blocks of all matter are atoms. Atoms are very small, they are smaller than anything that we can imagine or compare with.
Through the modern techniques, we can now produce magnified images of surfaces of elements showing atoms.
Dalton was the first scientist to use the symbols for elements in a very specific sense. When he used a symbol for an element he also meant a definite quantity of that element, that is, one atom of that element. Berzilius suggested that the symbols of elements be made from one or two letters of the name of the element.
Symbols for some elements as proposed by Dalton-:
|
Name of Elements |
Symbols of Elements |
|
Hydrogen |
⊙ |
|
Carbon |
|
|
Oxygen |
|
|
Phosphorous |
|
|
Sulphur |
⊕ |
|
Iron |
|
|
Copper |
|
|
Lead |
|
|
Silver |
|
|
Gold |
|
|
Platina |
|
|
Mercury |
|
In the beginning, the names of elements were derived from the name of the place where they were found for the first time. For example, the name copper was taken from Cyprus.
Many of the symbols are the first one or two letters of the element's name in English. The first letter of a symbol is always written as a capital letter (uppercase) and the second letter as a small letter (lowercase).
For example
Hydrogen, H
Aluminium, Al and not AL
Cobalt, Co and not CO.
Symbols of some elements are formed from the first letter of the name and a letter, appearing later in the name. Examples are: (i) chlorine, Cl, (ii) zinc, Zn etc.
According Dalton's atomic theory, each element had a characteristic atomic mass. The theory could explain the law of constant proportions so well that scientists were prompted to measure the atomic mass of an atom.
Symbols for some elements
|
Element |
Symbol |
|
Aluminium |
Al |
|
Argon |
Ar |
|
Barium |
Ba |
|
Boron |
B |
|
Bromine |
Br |
|
Calcium |
Ca |
|
Carbon |
C |
|
Chlorine |
Cl |
|
Cobalt |
Co |
|
Copper |
Cu |
|
Fluorine |
F |
|
Gold |
Au |
|
Hydrogen |
H |
|
Iodine |
I |
|
Iron |
Fe |
|
Lead |
Pb |
|
Magnesium |
Mg |
|
Neon |
Ne |
|
Nitrogen |
N |
|
Oxygen |
O |
|
Potassium |
K |
|
Silicon |
Si |
|
Silver |
Ag |
|
Sodium |
Na |
|
Sulphur |
S |
|
Uranium |
U |
|
Zinc |
Zn |
However, in 1961 for a universally accepted atomic mass unit, carbon-12 isotope was chosen as the standard reference for measuring atomic masses. One atomic mass unit is a mass unit equal to exactly one-twelfth (1/12th) the mass of one atom of carbon-12.
The relative atomic masses of all elements have been found with respect to an atom of carbon-12.
The relative atomic mass of the atom of an element is defined as the average mass of the atom, as compared to 1/12th the mass of one carbon-12 atom.
Atomic masses of a few elements
|
Element |
Atomic Mass (u) |
|
Hydrogen |
1 |
|
Carbon |
12 |
|
Nitrogen |
14 |
|
Oxygen |
16 |
|
Sodium |
23 |
|
Magnesium |
24 |
|
Sulphur |
32 |
|
Chlorine |
35.5 |
|
Calcium |
40 |
Atoms of most elements are not able to exist independently. Atoms form molecules and ions.
A molecule is in general a group of two or more atoms that are chemically bonded together, that is, tightly held together by attractive forces. A molecule can be defined as the smallest particle of an element or a compound that is capable of an independent existence and shows all the properties of that substance. Atoms of the same element or of different elements can join together to form molecules.
The molecules of an element are constituted by the same type of atoms. Molecules of many elements, such as argon (Ar), helium (He) etc. are made up of only one atom of that element.
If 3 atoms of oxygen unite into a molecule, instead of the usual 2, we get ozone, O3. The number of atoms constituting a molecule is known as its atomicity.
Atomicity of elements
|
Type of Element |
Name |
Atomicity |
|
Non-Metal |
Argon |
Monoatomic |
|
Helium |
Monoatomic |
|
|
Oxygen |
Diatomic |
|
|
Hydrogen |
Diatomic |
|
|
Chlorine |
Diatomic |
|
|
Phosphorus |
Tetra-atomic |
|
|
Sulphur |
Poly-atomic |
Atoms of different elements join together in definite proportions to form molecules of compounds.
Molecules of Compounds
|
Compound |
Combining Elements |
Ratio by Mass |
|
Water (H2O) |
Hydrogen, Oxygen |
1:8 |
|
Ammonia (NH3) |
Nitrogen, Hydrogen |
14:3 |
|
Carbon dioxide (CO2) |
Carbon, Oxygen |
3:8 |
Compounds composed of metals and nonmetals contain charged species. The charged species are known as ions.
Ions may consist of a single charged atom or a group of atoms that have a net charge on them. An ion can be negatively or positively charged. A negatively charged ion is called an ‘anion’ and the positively charged ion, a ‘cation’. A group of atoms carrying a charge is known as a polyatomic ion.
Ionic compounds
|
Ionic Compound |
Constituting Elements |
Ratio by Mass |
|
Calcium oxide |
Calcium and oxygen |
5:2 |
|
Magnesium |
Magnesium and sulphur |
3:4 |
|
Sodium chloride |
Sodium and chlorine |
23:35.5 |
The chemical formula of a compound is a symbolic representation of its composition. The chemical formulae of different compounds can be written easily.
The combining power (or capacity) of an element is known as its valency. Valency can be used to find out how the atoms of an element will combine with the atom(s) of another element to form a chemical compound.
The valency of the atom of an element can be thought of as hands or arms of that atom.
The rules that you have to follow while writing a chemical formula are as follows:
The valencies or charges on the ion must balance.
When a compound consists of a metal and a non-metal, the name or symbol of the metal is written first. For example: calcium oxide (CaO), sodium chloride (NaCl), iron sulphide (FeS), copper oxide (CuO) etc., where oxygen, chlorine, sulphur are nonmetals and are written on the right, whereas calcium, sodium, iron and copper are metals, and are written on the left.
In compounds formed with polyatomic ions, the number of ions present in the compound is indicated by enclosing the formula of ion in a bracket and writing the number of ions outside the bracket. For example, Mg (OH)2. In case the number of polyatomic ion is one, the bracket is not required. For example, NaOH.
The simplest compounds, which are made up of two different elements are called binary compounds.
While writing the chemical formulae for compounds, we write the constituent elements and their valencies as shown below. Then we must crossover the valencies of the combining atoms.
1. Formula of hydrogen chloride
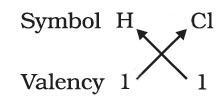
Formula of the compound would be HCl.
2. Formula of hydrogen sulphide
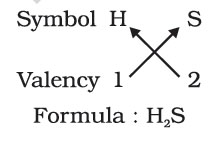
The molecular mass of a substance is the sum of the atomic masses of all the atoms in a molecule of the substance. It is therefore the relative mass of a molecule expressed in atomic mass units (u).
The formula unit mass of a substance is a sum of the atomic masses of all atoms in a formula unit of a compound. Formula unit mass is calculated in the same manner as we calculate the molecular mass. The only difference is that we use the word formula unit for those substances whose constituent particles are ions.
A chemical reaction equation indicates directly the number of atoms or molecules taking part in the reaction. Therefore, it is more convenient to refer to the quantity of a substance in terms of the number of its molecules or atoms, rather than their masses. So, a new unit “mole” was introduced.
One mole of any species (atoms, molecules, ions or particles) is that quantity in number having a mass equal to its atomic or molecular mass in grams.
The mass of 1 mole of a substance is equal to its relative atomic or molecular mass in grams. The atomic mass of an element gives us the mass of one atom of that element in atomic mass units (u).
The word “mole” was introduced around 1896 by Wilhelm Ostwald who derived the term from the Latin word moles meaning a ‘heap’ or ‘pile’. A substance may be considered as a heap of atoms or molecules. The unit mole was accepted in 1967 to provide a simple way of reporting a large number— the massive heap of atoms and molecules in a sample.
