The description of a chemical reaction in a sentence form is quite long. It can be written in a shorter form. The simplest way to do this is to write it in the form of a word-equation.
For example-: Described as − when a magnesium ribbon is burnt in oxygen, it gets converted to magnesium oxide.
Magnesium + Oxygen → Magnesium oxide
The magnesium and oxygen, are the reactants. The new substance is magnesium oxide, formed during the reaction, as a product.
A word-equation shows change of reactants to products through an arrow placed between them. The reactants are written on the left-hand side (LHS) with a plus sign (+) between them. Similarly, products are written on the right-hand side (RHS) with a plus sign (+) between them. The arrowhead points towards the products, and shows the direction of the reaction.
1.1.1 Writing chemical equations
A chemical equation represents a chemical reaction. If we recall the formulae of magnesium, oxygen and magnesium oxide, the above word-equation can be written as −
Mg + O2 → MgO
A chemical equation is a skeletal chemical equation for a reaction. This is a skeletal chemical equation for the burning of magnesium in air.
We have learnt that the mass can neither be created nor destroyed in a chemical reaction. The total mass of the elements present in the products of a chemical reaction has to be equal to the total mass of the elements present in the reactants.
The number of atoms of each element remains the same, before and after a chemical reaction. Hence, we need to balance a skeletal chemical equation.
The word-equation for Activity may be represented as −
Zinc + Sulphuric acid → Zinc sulphate + Hydrogen
The above word-equation may be represented by the following chemical equation −
Zn + H2SO4 → ZnSO4 + H2
The number of atoms of different elements on both sides of the arrow.
|
Element |
Number of atoms in reactants (LHS) |
Number of atoms in products (RHS) |
|
Zn |
1 |
1 |
|
H |
2 |
2 |
|
S |
1 |
1 |
|
O |
4 |
4 |
The chemical reactions involve the breaking and making of bonds between atoms to produce new substances.
A reaction in which a single product is formed from two or more reactants is known as a combination reaction.
For example-: Calcium oxide reacts vigorously with water to produce slaked lime (calcium hydroxide) releasing a large amount of heat.
CaO(s)/(Quick lime) + H2O(l) → Ca(OH)2(aq)/(Slaked lime) + Heat
In this reaction, calcium oxide and water combine to form a single product, calcium hydroxide.
When in chemical reaction a single reactant breaks down to give simpler products. This is a decomposition reaction.
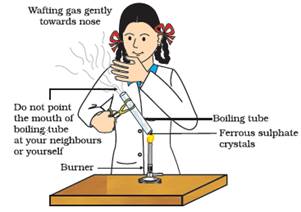
(Ferrous sulphate) 2FeSO4(s) → (Ferric oxide) Fe2O3(s) + SO2(g) + SO3(g)
The Ferrous sulphate crystals (FeSO4, 7H2O) lose water when heated and the colour of the crystals changes. It then decomposes to ferric oxide (Fe2O3), sulphur dioxide (SO2) and sulphur trioxide (SO3). Ferric oxide is a solid, while SO2 and SO3 are gases.
Decomposition of calcium carbonate to calcium oxide and carbon dioxide on heating is an important decomposition reaction used in various industries. Calcium oxide is called lime or quick lime. It has many uses − one is in the manufacture of cement. When a decomposition reaction is carried out by heating, it is called thermal decomposition.
When in chemical reaction react with some substance and it has displaced or removed another ne elements. This reaction is known as displacement reaction.
Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
In this reaction, iron has displaced or removed another element, copper, from copper sulphate solution.
A white substance, which is insoluble in water, is formed. This insoluble substance formed is known as a precipitate. Any reaction that produces a precipitate can be called a precipitation reaction
Na2SO4(aq) + BaCl2(aq) → BaSO4(s) + 2NaCl(aq)
The white precipitate of BaSO4 is formed by the reaction of SO2-4 and Ba2+. The other product formed is sodium chloride which remains in the solution. Such reactions in which there is an exchange of ions between the reactants are called double displacement reactions.
The surface of copper powder becomes coated with black copper(II) oxide. This is because oxygen is added to copper and copper oxide is formed.
2Cu + O2 → 2CuO
If hydrogen gas is passed over this heated material (CuO), the black coating on the surface turns brown as the reverse reaction takes place and copper is obtained.
CuO + H2 → Cu + H2O
If a substance gains oxygen during a reaction, it is said to be oxidised.
If a substance loses oxygen during a reaction, it is said to be reduced.
When a metal is attacked by substances around it such as moisture, acids, etc., it is said to corrode and this process is called corrosion. The black coating on silver and the green coating on copper are other examples of corrosion.
Corrosion causes damage to car bodies, bridges, iron railings, ships and to all objects made of metals, specially those of iron.
When fats and oils are oxidised, they become rancid and their smell and taste change. Usually substances which prevent oxidation (antioxidants) are added to foods containing fats and oil. Keeping food in air tight containers helps to slow down oxidation.
