The matter is made up of particles.
There must be millions of tiny particles in just one crystal of potassium permanganate, which keep on dividing themselves into smaller and smaller particles.
The particles of matter are very small — they are small beyond our imagination!!!
When we make tea, coffee or lemonade (nimbu paani ), particles of one type of matter get into the spaces between particles of the other. This shows that there is enough space between particles of matter.
Particles of matter are continuously moving, that is, they possess what we call the kinetic energy. As the temperature rises, particles move faster. So, we can say that with increase in temperature the kinetic energy of the particles also increases.
The particles of matter have force acting between them. A force keeps the particles together. The strength of this force of attraction varies from one kind of matter to another.
We can see that matter around us exists in three different states— solid, liquid and gas. These states of matter arise due to the variation in the characteristics of the particles of matter.
We can observe that the solid matter those all these have a definite shape, distinct boundaries and fixed volumes, that is, have negligible compressibility. Solids have a tendency to maintain their shape when subjected to outside force. Solids may break under force but it is difficult to change their shape, so they are rigid.
The liquids have no fixed shape but have a fixed volume. They take up the shape of the container in which they are kept. Liquids flow and change shape, so they are not rigid but can be called fluid.
The gases are highly compressible as compared to solids and liquids. The liquefied petroleum gas (LPG) cylinder that we get in our home for cooking or the oxygen supplied to hospitals in cylinders is compressed gas. Compressed natural gas (CNG) is used as fuel these days in vehicles. Due to its high compressibility, large volumes of a gas can be compressed into a small cylinder and transported easily.
In the gaseous state, the particles move about randomly at high speed. Due to this random movement, the particles hit each other and also the walls of the container.
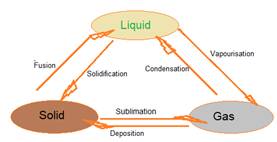
On increasing the temperature of solids, the kinetic energy of the particles increases. Due to the increase in kinetic energy, the particles start vibrating with greater speed. The energy supplied by heat overcomes the forces of attraction between the particles. The particles leave their fixed positions and start moving more freely. A stage is reached when the solid melts and is converted to a liquid. The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point.
The melting point of a solid is an indication of the strength of the force of attraction between its particles.
The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point. Boiling is a bulk phenomenon. Particles from the bulk of the liquid gain enough energy to change into the vapour state.

So, we infer that the state of matter can be changed into another state by changing the temperature.
A change of state directly from solid to gas without changing into liquid state is called sublimation and the direct change of gas to solid without changing into liquid is called deposition.
Applying pressure and reducing temperature can liquefy gases.
Solid CO2 gets converted directly to gaseous state on decrease of pressure to 1 atmosphere* without coming into liquid state.
We can say that pressure and temperature determine the state of a substance, whether it will be solid, liquid or gas.
We know that particles of matter are always moving and are never at rest. At a given temperature in any gas, liquid or solid, there are particles with different amounts of kinetic energy. In the case of liquids, a small fraction of particles at the surface, having higher kinetic energy, is able to break away from the forces of attraction of other particles and gets converted into vapour.
The phenomenon of change of a liquid into vapours at any temperature below its boiling point is called evaporation.
The rate of evaporation increases with—
An increase of surface area: If the surface area is increased, the rate of evaporation increases.
An increase of temperature: With the increase of temperature, more number of particles get enough kinetic energy to go into the vapour state.
A decrease in humidity: Humidity is the amount of water vapour present in air. The air around us cannot hold more than a definite amount of water vapour at a given temperature.
An increase in wind speed: It is a common observation that clothes dry faster on a windy day. With the increase in wind speed, the particles of water vapour move away with the wind, decreasing the amount of water vapour in the surrounding.
In an open vessel, the liquid keeps on evaporating. The particles of liquid absorb energy from the surrounding to regain the energy lost during evaporation. This absorption of energy from the surroundings make the surroundings cold.
The heat energy equal to the latent heat of vaporisation is absorbed from the body leaving the body cool.
